Genetically Encoded Gas Vesicles as Ultrasound Contrast Agents
A range of bacteria and archaea produces intracellular gas-filled protein structures that function as flotation devices to maintain a suitable depth in water. GVs are perfect contrast agents for ultrasound due to high impedance mismatch. GVs are found in cyanobacteria, halophilic archaea, and Bacillus megaterium (MegaGV). Baillus megaterium gas vesicle protein (Gvp) was identified and successfully transferred to E.coli. Mammalian cell expression was also demonstrated. The wall of GVs is freely permeable to gas molecules and is composed of a small hydrophobic protein, GvpA, which forms a single-layer wall. In addition, several minor structural accessory or regulatory proteins are required for GV formation. Spectral GV imaging can be achieved if different combinations of Gvps are used to generate different types of GVs that emits distinct resonance frequency under ultrasound excitation. In general, GvpA and its repeat with small variation, GvpB or GvpA1 or GvpA3, are main building blocks of the GV structure. Calothrix sp. PCC 7601 produce large and cylindrical shape GVs while Pseudanabaena sp. PCC 6901 produce smaller GVs. Inspired by the fact that distinct GVs are synthesized by different strains in cyanobacteria, multiplexed imaging to track mammalian cells may be possible by expressing unique GVs in each cell type.
This transfer of genes between species will demonstrate the exciting synthesis of a functional organelle. The success of this study will provide an opportunity for the generation of GV and its variants using different Gvp gene clusters in E.coli and mammalian cells. Isolated GVs with chemical conjugation of functional moieties on their surface and genetically encoded biomarkers combined with Gvp genes for GV expression in mammalian cells will open a new avenue for imaging and therapeutic applications. Developed GV gene circuits can be used to study the GV’s molecular constitution and the regulation of GV synthesis. Smaller size of gene circuits for GV expression in mammalian cells reduce the burden for in vivo delivery and even in vitro engineering of primary cells using intracellular delivery techniques such as viral vector and electroporation.
Further reading:
1. Kim, S., Zhang, S., Yoon, S.*, (2021). Multiplexed ultrasound imaging using clustered gas vesicles and spectral imaging. bioRxiv. https://biorxiv.org/cgi/content/short/2021.10.15.464596v1
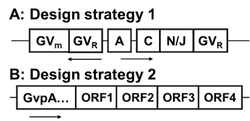
Figure 1 shows gvp gene clusters that is used for heterologous GV expression.
This transfer of genes between species will demonstrate the exciting synthesis of a functional organelle. The success of this study will provide an opportunity for the generation of GV and its variants using different Gvp gene clusters in E.coli and mammalian cells. Isolated GVs with chemical conjugation of functional moieties on their surface and genetically encoded biomarkers combined with Gvp genes for GV expression in mammalian cells will open a new avenue for imaging and therapeutic applications. Developed GV gene circuits can be used to study the GV’s molecular constitution and the regulation of GV synthesis. Smaller size of gene circuits for GV expression in mammalian cells reduce the burden for in vivo delivery and even in vitro engineering of primary cells using intracellular delivery techniques such as viral vector and electroporation.
Further reading:
1. Kim, S., Zhang, S., Yoon, S.*, (2021). Multiplexed ultrasound imaging using clustered gas vesicles and spectral imaging. bioRxiv. https://biorxiv.org/cgi/content/short/2021.10.15.464596v1
Figure 1 shows gvp gene clusters that is used for heterologous GV expression.